Publications and News
May 2024 – Second call for applications to assess efficacy of Mycobacterium tuberculosis vaccine candidates in a mouse model
Diversification of the vaccine development pipeline has been identified as a priority area in the Global TB Vaccine R&D Roadmap. The TBVAC-HORIZON consortium aims, amongst other things, to fill the early phases of the development pipeline while ensuring a diverse portfolio.
One of the objectives of the project is to perform head-to-head comparisons of new candidate TB vaccines both within and outside of the consortium.
We are pleased to announce our second call for applications. This call will grant access to a head-to-head assessment of candidate vaccines. The assessment will evaluate the ability of vaccines to reduce bacterial load using a standard mouse model of vaccination and pulmonary challenge with Mycobacterium tuberculosis.
A total of four calls are foreseen within the TBVAC-HORIZON project. In the current round, there is room to asses up to six vaccine candidates in this mouse model, and we invite all who have a candidate in a relevant stage of development to submit your vaccine candidate for testing in this model.
For more information, please read the guidance along with supporting information and download the application form..
April 2024 – University of Oxford (UOXF) starts aligned clinical study – first participant enrolled
TBVAC-HORIZON has met an important milestone: On 3 April 2024, the first participant in the UOXF “TB46” study was enrolled.
Tuberculosis (TB) remains one of the most devastating infectious diseases worldwide, killing over 4,000 people every day. Prevention of TB infection by novel vaccines would provide the most cost-effective approach to achieve the goals of the WHO’s End TB strategy and the UN’s Sustainable Development Goals. While there are few promising TB vaccine candidates available, innovation by new platforms and strategies is needed to ensure that the most effective and affordable vaccines are developed.
The Horizon Europe-funded project TBVAC-HORIZON will improve understanding of lung immunity in tuberculosis in order to establish a diverse and innovative global TB vaccine pipeline targeting mucosal immunity.
One of the objectives is to evaluate whether mucosal revaccination with BCG (the current and only licensed vaccine against TB) improves protective efficacy against infection with Mtb in aligned studies with humans, non-human primates and small animals (mice). The Oxford study will compare whether administering BCG via an inhaled (lung) route stimulates a stronger and better immune response than administering it under the skin (the conventional, intradermal route). As the natural route of infection with TB is through inhalation of droplets into the lungs, this approach is thought to be better at stimulating the immune system. Also it will be compared whether adminstration of BCG intradermally to individuals with Type 2 Diabetes will stimulate as strong an immune response as in healthy persons without Diabetes. This is an important aspect as people with diabetes are more likely to develop TB disease as well as develop complications from it: Better understanding of the immune response to BCG in immunocompromised individuals is urgently needed.
In this study, BCG will be administered for a second time to persons vaccinated with BCG once before. In total, 36 healthy volunteers aged between 18 and 65 will be recruited (of which 12 with type 2 Diabetes), and divided in 3 groups of 12 participants. The study is planned for 24 months, and its results are expected by the end of TBVAC-HORIZON in 2027.
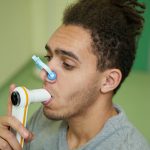 Pict. 1 Breathing into a lung function machine. Lung function tests are done at the screening visit and after BCG vaccination
Pict. 1 Breathing into a lung function machine. Lung function tests are done at the screening visit and after BCG vaccination
Pict. 2 Volunteer receiving aerosol BCG vaccination in a negative pressure tent
February 2024 – The TBVAC-HORIZON consortium met in Les Diablerets
The first annual progress meeting of TBVAC-HORIZON was held from January 30 to February 1, 2024, in Les Diablerets, Switzerland. All consortium partners provided updates on the developments and progress made during the project’s first year.
In specific sessions members discussed their work packages’ progress. A workshop on panel aligment for Flow Cytometry was held. Results were presented in the plenary session, in which the advisory committee members also attended. On the last day, the Scientific Advisory and Ethics Advisory boards met. The Portfolio Advisory Committee also met to review the applications to assess novel Mycobacterium tuberculosis vaccine candidates for their efficacy head-to-head in mice. In the subsequent Steering Committee meeting, the Advisory Boards presented their evaluation outcomes.
All attendees also attended the TBVI symposium on January 30-31, in Les Diablerets as well. This offered a great chance to exchange knowledge and explore new initiatives with other scientists, policy makers and funders within and outside the TB field.
We look back on a very informative and motivational week. TBVI is grateful to all the participants for their enthusiastic attendance and valuable contributions which led to the triumph of the event. TBVI is eagerly looking forward to continue making TBVAC-HORIZON’s outcomes a meaningful impact in the fight against TB.
October 2023 – Call for applications to assess efficacy of Mycobacterium tuberculosis vaccine candidates in a mouse model
Diversification of the vaccine development pipeline has been identified as a priority area in the Global TB Vaccine R&D Roadmap. The TBVAC-HORIZON consortium has been able to secure funds for a 4-year project that aims, amongst other things, to fill the early phases of the development pipeline while ensuring a diverse portfolio. One of the objectives of TBVAC-HORIZON is to perform head-to-head comparisons of new candidate TB vaccines both within and outside of the consortium.
To this aim, we are delighted to announce the first call for applications that will grant access to a head-to-head assessment of the ability of candidate vaccines to reduce bacterial load. This assessment will be performed using a standard mouse model of vaccination and pulmonary challenge with Mycobacterium tuberculosis. Please read the TBVAC Horizon call for application as well as supporting information and guidance for the application and the TBVAC-HORIZON Application form.
A total of three calls are foreseen within the TBVAC-HORIZON project. In the current first round, there is room to asses up to three vaccine candidates in this mouse model, and we invite all who have a candidate in a relevant stage of development to submit your vaccine candidate for testing in this model by completing the application form attached and send this to info@tbvi.eu before 1 January 2024.
April 2023 – TBVAC-HORIZON kicks off
The Horizon Europe programme has funded the TBVAC-HORIZON project to improve the understanding of lung immunity to tuberculosis infection and to establish a diversified innovative TB vaccine pipeline targeting mucosal immunity. TBVAC-HORIZON, consisting of 19 partners, will be coordinated by TBVI and will run for 4 years.





