In its strategy, TBVI considers the need to: discover and develop safe and effective vaccines for all; prevent infection and transmission of disease; build on the R&D comparative advantage of the TBVI consortium members; make the best use of limited resources and mobilise additional funding.
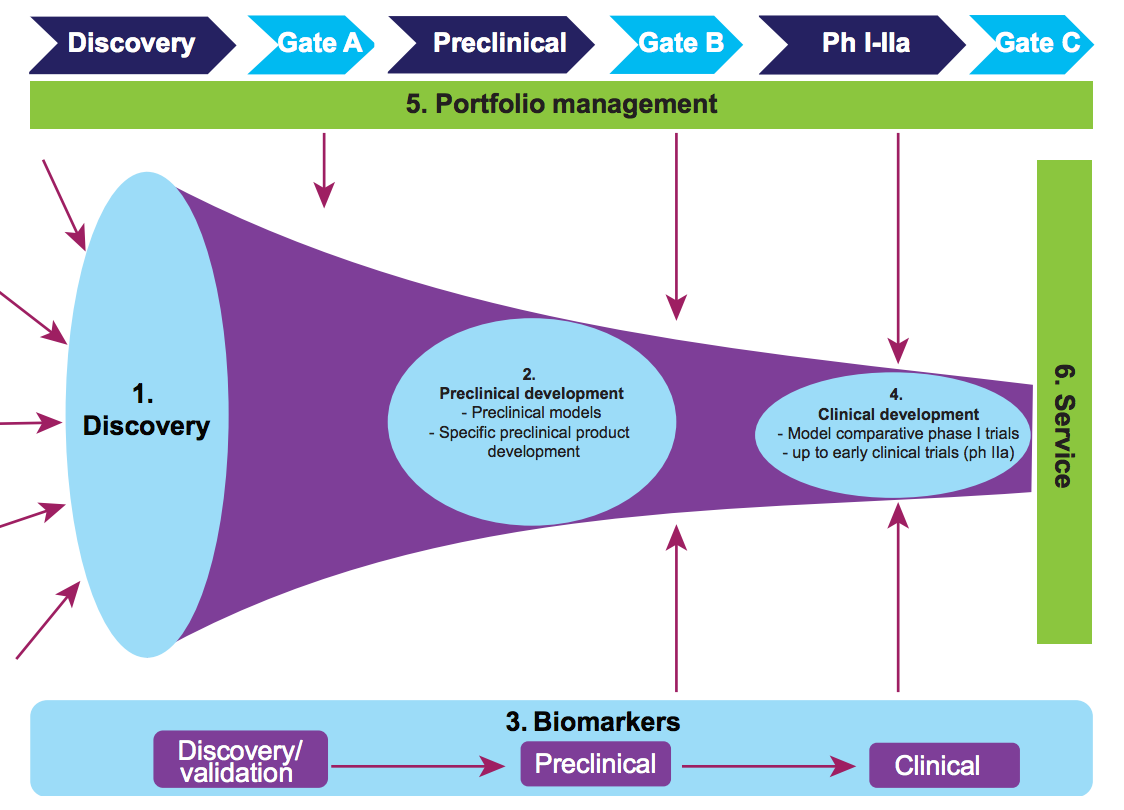
TBVI has prioritised the following R&D areas to meet its objectives (see figure 1).
Figure 1: TBVI focus areas
Discovery
TBVI focuses on innovating, expanding and diversifying the TB vaccine pipeline. This is based on cutting-edge research addressing knowledge gaps in immune-mediated protection against TB infection and disease, and on new vaccine concepts targeting diverse immune mechanisms.
TBVI supports:
- new antigen discovery, including protein and non-protein (e.g. glycolipid) targets;
- novel formulations and delivery systems;
- alternative routes and methods of vaccine administration;
- development of safer and more effective live vaccines.
Preclinical development
New priming and boosting vaccines
The BCG vaccine, available since 1921, can protect children from severe forms of tuberculosis. However, BCG has little to no efficacy in preventing pulmonary TB in adolescents and adults, the most common and most infectious form of tuberculosis. Therefore, efforts are being undertaken to improve or replace BCG with better vaccines. While appreciating that the greatest impact on transmission is by developing vaccines that target adolescents and adults, efforts to develop new and safer priming vaccines for infants will also be supported.
TBVI supports:
- development of priming vaccines that perform better and/or are safer than BCG;
- development of booster vaccines;
- development of prime-boost strategies;
- development of post-infection vaccine strategies for therapeutic use;
- comparative testing of new vaccines in standardised (preclinical) models;
- GMP manufacturing, and toxicity and safety studies.
Preclinical models
The development of new vaccines is dependent upon robust preclinical animal models in order to select those vaccine candidates, which should progress to clinical development. These standardised animal models and their read-outs provide evidence of safety, immunogenicity and/or protective efficacy of new vaccine candidates. Preclinical data then support portfolio management and decision-making based on predefined gating and priority setting criteria.
To improve and extend beyond its current preclinical portfolio of models, TBVI supports the refinement of existing models and the development of others specifically fit-for-purpose in several species. Refined non-human primate experiments remain a valuable bridging tool between preclinical and clinical development. TBVI and its partners comply with the highest ethical standards for using animals in TB vaccine development, where no suitable alternatives are available.
TBVI supports:
- standardisation, harmonisation and refinement of preclinical models;
- comparative (head-to-head) testing and immunological evaluation;
- models to evaluate prime-boost strategies;
- post-exposure vaccination models;
- exchange of (clinical and) preclinical data to support R&D into correlates of protection.
Biomarkers
Better correlates of protection will help to identify relevant antigens, to develop improved vaccines and to allow the demonstration of their immunogenicity and potential efficacy at an early stage. Importantly, such correlates will facilitate the selection of candidate TB vaccines, and accelerate and reduce the cost of human efficacy trials. In addition, these correlates will permit optimisation of dose, vehicle, adjuvants, formulations and immunisation schedules for new candidate vaccines at an early stage, and thereby inform preclinical study design. Correlates of disease risk will be invaluable in the stratification of individuals in clinical studies, contributing to the optimisation of these studies.
TBVI supports:
- biomarker discovery;
- development and standardisation of biomarker assays;
- translation and validation of biomarkers in TB vaccine trials.
Clinical Development
TBVI aims to support and accelerate promising candidates through early clinical development. TBVI aims to incorporate new developments in clinical trial design.
TBVI supports:
- clinical experimental medicine studies;
- development of a human challenge model for TB;
- first-in-man (Phase I) clinical trials and Phase IIa trials;
- the planning, guidance and evaluation of Phase IIb and Phase III trials.
Portfolio management
Portfolio management is considered to be an efficient and effective mechanism to advance a vaccine pipeline. It is a structured and evidence-based decision-making process that seeks to maximise the probability of success against acceptable cost and risk. It is built around agreed criteria for success at the various stages of vaccine development and an objective assessment of the data against those criteria. TBVI applies the portfolio management process at entry, stage gating and priority setting of candidates.
TBVI, together with IAVI, developed and applies this transparent quality decision-making process that allows for fact-based, data-driven and strategy-oriented management decisions. This process is built on four pillars to ensure strategic alignment of vaccine candidates and resources, and an optimal balance between them:
- filling the pipeline with new vaccine candidates and regimes, governed by a set of entry criteria: to encourage entry of new candidates into the inventory and to ensure diversity and complementarity
- stage gating: a framework to aid decision-making in advancing and investing further in candidates through the R&D pipeline
- priority setting: a matrix process to prioritise candidates competing for available resources
- resource mobilisation and fund management
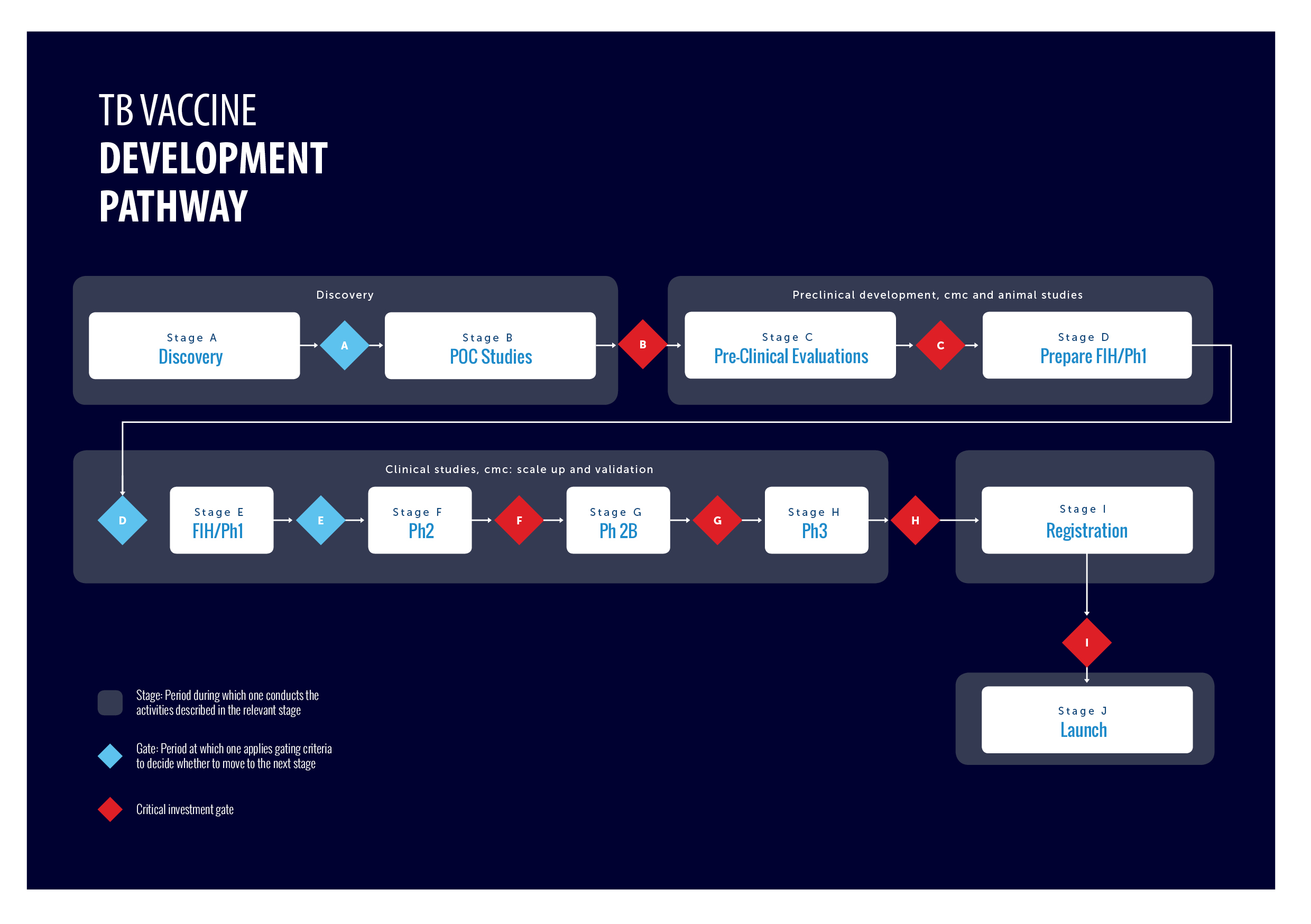
TB Vaccine development pathway
In the effort to eradicate tuberculosis as one of the world’s most deadly diseases, novel TB vaccines will be an important part of the solution. There is a pipeline of new TB vaccines that requires a global and comprehensive coordination of efforts with defined stages of development and criteria for progression of individual vaccine candidates.
To address this, the TB Vaccine Development Pathway is an established tool that provides a structured development path and gating criteria for TB vaccine candidates. It also describes the different functions and capabilities required to advance a candidate TB vaccine to its next stage of development.
The Pathway was developed by experts from TBVI and IAVI, and consolidated with a variety of stakeholders in the field of TB vaccines. It was developed on behalf of the Global TB Vaccine Partnership (GTBVP), an alliance of organisations that aim at making novel TB vaccines a reality, with funding from the Bill & Melinda Gates Foundation.
The development of vaccines can be divided into stages, from discovery to implementation. The decision to move a vaccine candidate to the next stage is difficult, and even more so for TB vaccines because of the lack of a correlate of protection, of predictive animal models, of precedence for obtaining market authorization, and the restricted amount of funding available.
The TB Vaccine Development Pathway serves as a guidance tool to help developers consider all the functions of development and how they must work together to advance a vaccine to its next stage of development.
Please visit the Pathway at www.TBVacPathway.org and / or read the article published in Tuberculosis The TB vaccine development pathway – An innovative approach to accelerating global TB vaccine development.