TBVI is continuously working on supporting the development of new vaccine candidates. Vaccine developers are kindly invited to share an update if needed by contacting TBVI. Please note that the overview of candidates in the preclinical phase is not complete. These are all preclinical candidates in which TBVI is involved. Please also consult the website Working Group on New TB Vaccines.
The current TB vaccine pipeline (last update January 2024), is as follows:
-
Live
-
Wholecell
-
Subunit
-
Vector
-
TBVI involved
-
RNA
TBVI provides technical support for product and clinical development to TB vaccine researchers and developers.
TBVI has initiated and coordinated the realisation of a joint publication regarding preferred product characteristics (PPC) for therapeutic vaccines to improve tuberculosis treatment outcomes. This PPC is a result of a consensus generating consultation process from the WHO, TBVI and IAVI with input from experts within the TB field and beyond. To read the full publication, please follow the link: “Preferred product characteristics for therapeutic vaccines to improve tuberculosis treatment outcomes: Key considerations from World Health Organization consultations”.
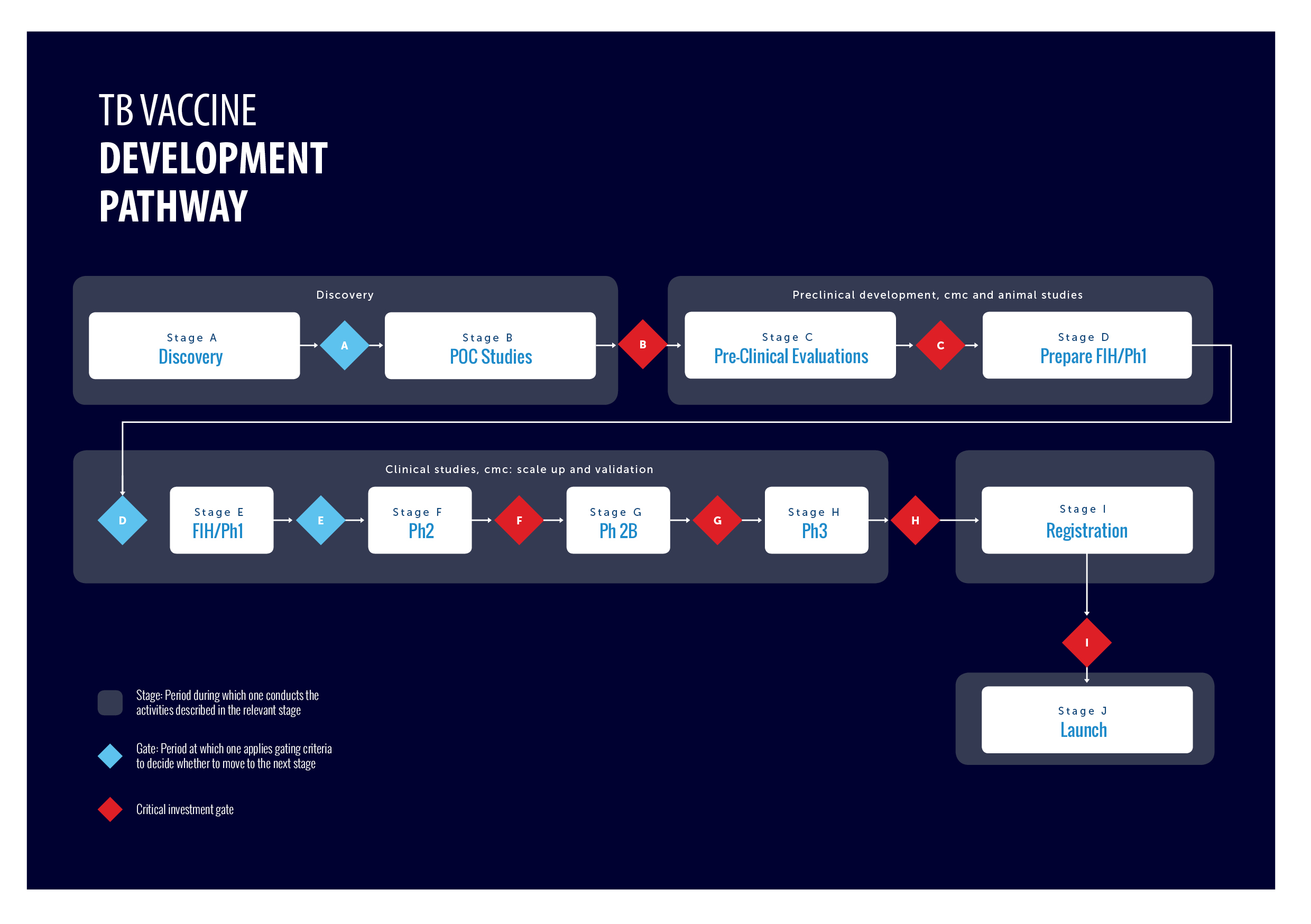
TB Vaccine development pathway
The TB vaccine pipeline requires a global and comprehensive coordination of efforts with defined stages of development and criteria for progression of individual vaccine candidates. To address this, the TB Vaccine Development Pathway is an established tool that provides a structured development path and gating criteria for TB vaccine candidates. It also describes the different functions and capabilities required to advance a candidate TB vaccine to its next stage of development.
For more information about the Pathway, please visit www.TBVacPathway.org , read the article published in Tuberculosis The TB vaccine development pathway – An innovative approach to accelerating global TB vaccine development and/or Stage Gating Criteria Tables 2021
 For more information, please contact Marit Holleman.
For more information, please contact Marit Holleman.

