TBVAC-HORIZON: Innovating and diversifying the TB vaccine pipeline
TBVAC-HORIZON: Innovating and diversifying the TB vaccine pipeline
Aim and objectives
While there are few vaccine candidates in late-stage clinical trials, the TB vaccine pipeline remains insufficient and needs diversification and innovation. To ensure that the most effective and affordable vaccines are developed, innovation by new platforms and strategies is needed.
TBVAC-HORIZON will address this through in-depth investigation of the mechanisms of immune responses to infection in the lung, which will identify biomarkers to rationalise vaccine design and improve monitoring of vaccine immunity.
The translational component of the project includes head-to-head comparison of novel candidate vaccines in standardised animal models, aligned comparative experimental medicine studies in humans and non-human primates, and assessment of immune responses in individuals with comorbidity-induced increased susceptibility to TB. It will also establish novel delivery systems and adjuvant formulations. Finally, a novel GMP platform for live attenuated vaccines will be developed.
The combination of basic, applied and translational research, preclinical efficacy and human experimental medicine studies, improved adjuvants, and novel GMP platforms will pave the way for novel concepts for candidate vaccines and improved immunisation strategies with the ultimate goal of accelerated availability of affordable, accessible and more effective TB vaccines.
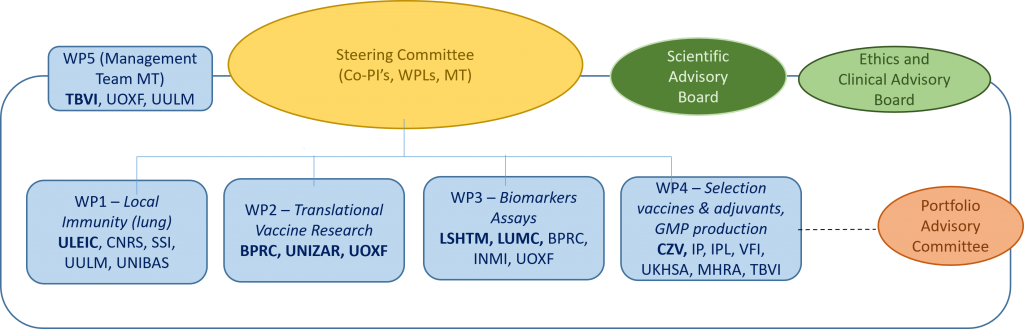
Work packages
TBVAC-HORIZON is divided into five work packages:
- WP 1: Mechanisms of immune protection in the M. tb-infected lung
- WP 2: Improving lung immunity by mucosal vaccine delivery (Translational Vaccine Research)
- WP 3: Identifying biomarker assays indicating vaccine-induced protection
- WP 4: Innovating expression, formulation, evaluation and GMP tools for next generation TB vaccine development
- WP 5: Project coordination
Project governance:
The consortium will be advised by the Scientific Advisory Board, the Ethics and Clinical Advisory, and the Portfolio Advisory Committee.