Our research
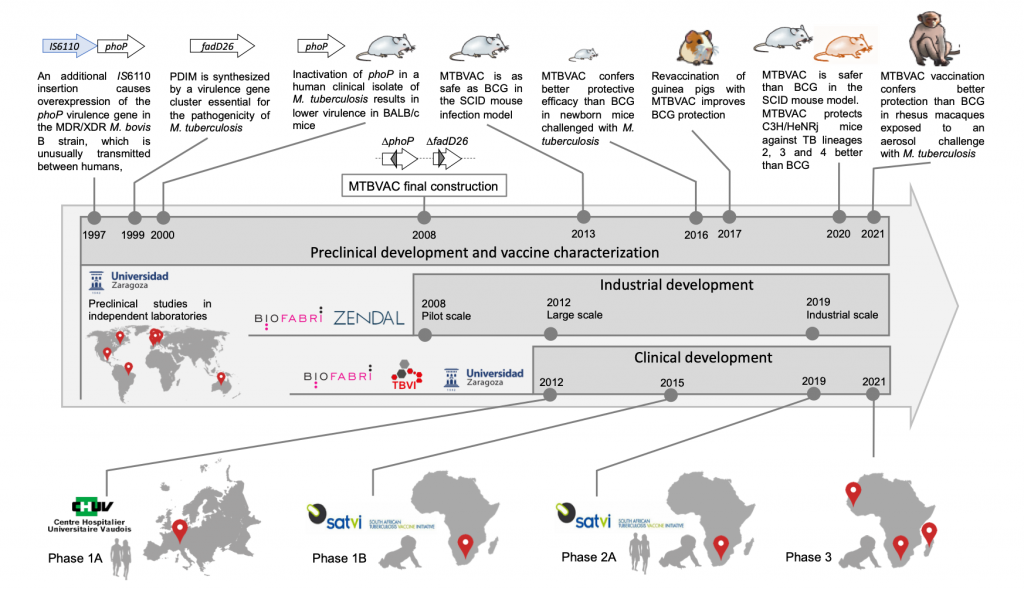
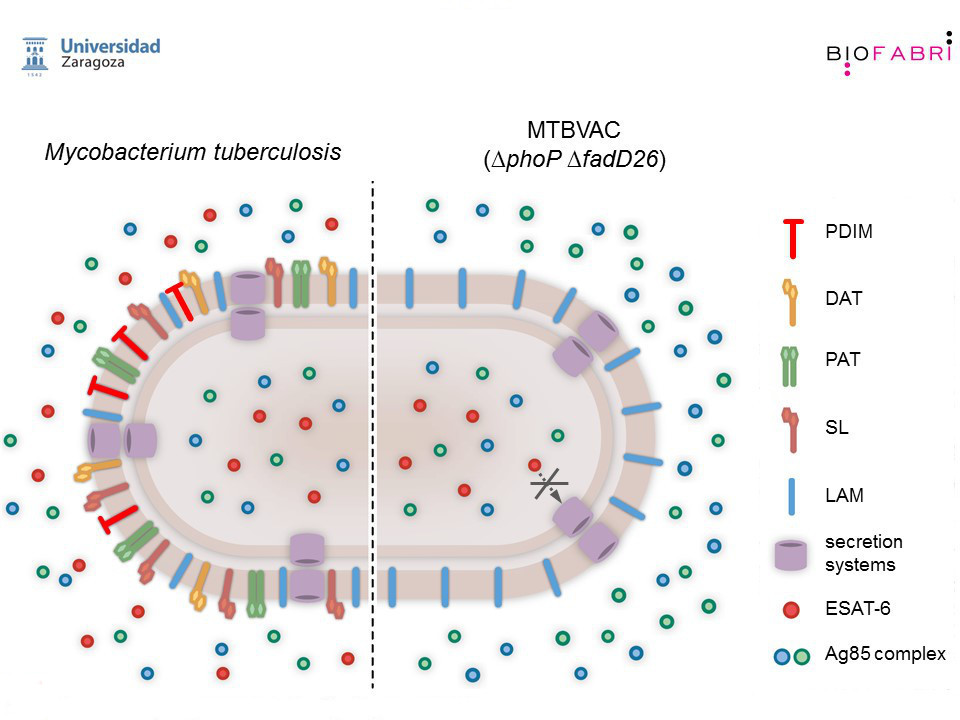
We have led the research and discovery of the live vaccine candidate MTBVAC, which is based on two stable deletion mutations, without antibiotic resistance markers, in the virulence genes phoP and fadD26 engineered in a clinical strain of Mycobacterium tuberculosis. Inside the TBVI consortium, the discovery phase of MTBVAC has included rigorous preclinical characterization by independent laboratories and research partners demonstrating robust attenuation, safety, and immunogenicity and protective efficacy profiles in different animal models supporting further vaccine development.
The promising preclinical data led UZ to form an industrial partnership with the Spanish biopharmaceutical company Biofabri with the objective to manufacture and develop MTBVAC as vaccine for human use (in compliance with Good Manufacturing Practices) supporting progress to first in human clinical evaluation. As result of the successful industry – academia collaboration coupled with the help of independent expert advisors through TBVI PDT/CDT, in Oct 2013, MTBVAC entered first-in-human clinical evaluation Phase 1a in adults at CHUV in Lausanne, Switzerland (NCT02013245) evaluating safety, tolerability and immugenicity of MTBVAC as compared to BCG. The trial successfully completed in Nov 2014 (Spertini et al 2015 Lancet Respir Med).
Following the first-in-human Phase 1a trial, MTBVAC was evaluated in a dose-escalation Phase 1b safety and immunogenicity trial in South Africa in its primary target age group population, namely healthy HIV-unexposed newborn babies. Conducted by the South African Tuberculosis Vaccine Initiative (SATVI).
Phase 1b trial included a safety arm in healthy adults as a safety step prior to vaccination of newborn babies. MTBVAC Phase 1b trial confirmed vaccine safety and a greater immunogenicity as compared to the same dose of BCG SSI, suggestive of the broader antigenic content (Tameris et al Lancet Respir Med 2019).
MTBVAC is now completing two dose-defining Phase 2 trials in South Africa at SATVI, using BCG as reference comparator. A Phase 2a in newborns (NCT03536117) and a Phase 1b/2a in BCG vaccinated adolescents and adults with and without prior M. tuberculosis infection (NCT02933281). The vaccination phase has been completed and safety and immunogenicity results will allow the dose-definition of MTBVAC for entry into already planned multicenter Phase 3 efficacy evaluation in newborns in Sub-Saharan Africa. MTBVAC project was recently awarded partial funding through The European & Developing Countries Clinical Trials Partnership (EDCTP2) to conduct a multi-center Phase 3 efficacy trial in newborn babies in South Africa, Madagascar and Senegal, which is now estimated to initiate in 2022. The promising safety and immunogenicity data support clinical development of MTBVAC in different target age-group populations (from adults to newborns) in TB-endemic countries (NCT04975178). Biofabri is the Clinical Trial Sponsor of MTBVAC in collaboration with University of Zaragoza (Spain), TBVI and IAVI, partner to advance the tuberculosis vaccine candidate MTBVAC into efficacy trials.
Our group
The Mycobacterial Genetics Research Group, at the University of Zaragoza was founded by Carlos Martin in 1992 after his return from Professor Brigitte Gicquel’s lab at Pasteur Institute in Paris, where he was a permanent researcher for five years. Carlos Martin MD, PhD is a Professor of Microbiology in the Faculty of Medicine at University of Zaragoza and member of the TBVI Steering Committee, with more than 30 years of experience in mycobacterial genetics.
Since 1992 the group has led numerous pioneering TB research projects, both of national and international significance, in TB diagnosis, drug resistance and vaccine discovery using state-of-the-art M. tuberculosis genetic engineering. In the sphere of TB vaccine discovery, our team aims to develop novel tuberculosis vaccines and vaccination strategies to improve protection against pulmonary TB. Current work is being done in collaborative tuberculosis research projects together with research groups of Europe (TBVAC2020) and Latin America (EurolacTB and SLAMTB). Since 2007 our group has been integrated into the Spanish Research Network on Respiratory Disease, CIBERES, (Instituto de Salud Carlos III).
MTBVAC Clinical trial
“Dose-Escalation Study to Evaluate the Safety and Immunogenicity of MTBVAC Vaccine in Comparison With BCG Vaccine”
(ClinicalTrials.gov : NCT02013245)
“Dose-escalation Safety and Immunogenicity Study to Compare MTBVAC to BCG in Newborns With a Safety Arm in Adults (MTBVAC-Ph1b)”
(ClinicalTrials.gov : NCT02729571)
“MTBVAC Study in Adults With and Without Latent Tuberculosis Infection in South Africa”
(ClinicalTrials.gov NCT02933281 )
“Dose-Defining Safety and Immunogenicity Study of MTBVAC in South African Neonates”
(ClinicalTrials.gov: NCT03536117)
“Efficacy, Safety and Immunogenicity Evaluation of MTBVAC in Newborns in Sub-Saharan Africa (MTBVACN3)”
(ClinicalTrials.gov Identifier: NCT04975178)
Projects
EDCTP2 (RIA2016V-1637). MTBVAC in Newborns / MTBVAC in Newborns: Phase 2a Dose-Defining Safety and Immunogenicity Study and Capacity Building to Support Vaccine Efficacy Trials in Tuberculosis-Endemic Regions of Sub-Saharan Africa. For more information, visit the project webpages.
2018- 2022
EDCTP 3 (RIA2019S-2652). MTBVACN3 – MTBVAC in Newborns – Phase 3 / Randomised, double blind controlled phase 3. Trial to evaluate the efficacy, safety and immunogenicity of MTBVAC administered in healthy HIV unexposed uninfected and HIV exposed uninfected newborns in Tuberculosis endemic regions of Subsaharian Africa. 2021-2026
RTI2018-097625-B-10. Spanish Ministry: Science and Innovation. Retos Sociedad. “Study of the trained innate immunity conferred by the live attenuated BCG and MTBVAC vaccines, and their application for therapeutic use in diseases with immunological content”. 2019-2022
RTC-2017. 379-1. Spanish Ministry Science and Innovation. Retos Colaboración. “Improved treatment of bladder cancer by Bacilotherapy”. Retos Colacoración Institutions involved: University of Zaragoza, BIOFABRI and CSIC (CNB). 2018-2021.