MTBVAC is a novel TB vaccine candidate based on an attenuated M. tuberculosis clinical isolate. Safety and immunogenicity of MTBVAC was demonstrated in BCG naïve adults; and MTBVAC appears safe in a Phase 1b study in South African newborns. Definitive demonstration of safety and immunogenicity at the optimal MTBVAC dose is key to progression into multi-centre efficacy trials in infants.
EDCTP supports this Phase 2a dose-defining study of MTBVAC to evaluate the safety, reactogenicity, immunogenicity, and potential for IGRA conversion and reversion, of MTBVAC in South African newborns.
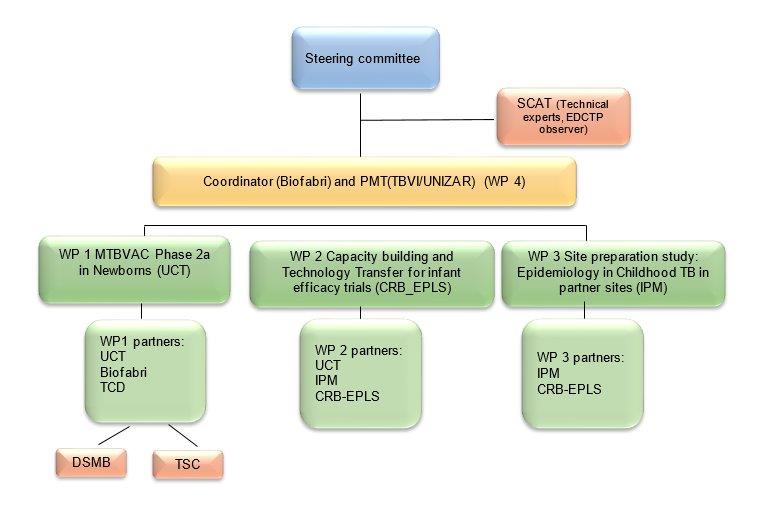
Governance structure
The MTBVAC-NEWBORN consortium is composed of 3 European and 3 sub-Saharan African participants. Two participants are Universities (UCT, UNIZAR), two are Higher Education Institutions (CRB-EPLS, IPM), one is a product development partnership, supporting and coordinating TB vaccine development (TBVI), and one is private industry (BIOFABRI).
Coördinator and partners

Biofabri focuses on two strategic lines of business: on the one hand, the development and biological production of vaccines and, on the other, the manufacturing and distribution of biopharmaceutical specialties and biotech services for third parties.
Amongst the most advanced projects in its pipeline is the promising vaccine candidate against Tuberculosis, MTBVAC.
The MTBVAC vaccine, developed and manufactured entirely in Spain, is one of the most promising vaccines against tuberculosis in the portfolio of vaccines candidates. MTBVAC is a live attenuated freeze-dried vaccine developed by Biofabri, from a strain designed by the research group of Carlos Martin of the University of Zaragoza (Spain) next to the Pasteur Institute
Biofabri as Coordinator within the Global Consortium:
- The Coordinator shall be the intermediary between the beneficiaries and the EDCTP Association and shall perform all tasks assigned to it as described in the Grant Agreement
In particular the Coordinator shall be responsible for:
- Monitoring compliance by the Parties with their obligations
- Keeping the address list of Members and other contact persons updated and available
- Collecting, reviewing to verify consistency and submitting reports, other deliverables (including financial statements and related certifications) and specific requested documents to the Funding Authority
- Transmitting documents and information connected with the Project to any other Parties concerned
- Administering the financial contribution of the Funding Authority and fulfilling the financial tasks
- Providing, upon request, the Parties with official copies or originals of documents that are in the sole possession of the Coordinator when such copies or originals are necessary for the Parties to present claims.

UNIZAR as the discoverer and initial developer of MTBVAC, will provide its longstanding preclinical, immunological and scientific expertise to advise as part of the Scientific and Clinical Advisory Team (SCAT) on the proposed clinical, capacity building, epidemiological activities, and to provide scientific support to the project management activities. UNIZAR will also provide key support in regularly updates of the Investigational Brochure of MTBVAC in response to the outcomes of the activities of this project.

TBVI provides its longstanding project management and coordination expertise to support the coordinator (Biofabri) in its role to coordinate and manage the project.
TBVI also provides its regulatory and clinical expertise to advise as part of SCAT on the proposed clinical, capacity building, and epidemiological activities.
The South African Tuberculosis Vaccine Initiative (SATVI) is a tuberculosis (TB) research group with a research scope that spans several disciplines including paediatrics, infectious diseases, epidemiology, public health, immunology, systems biology and clinical sciences.
SATVI has a large and well-developed clinical field site in the Cape Winelands East region, with the core facility on the premises of the Brewelskloof TB Hospital in Worcester, from where most clinical/epidemiological studies and clinical trials are conducted.

CRB-EPLS, Centre de Recherche Biomedicinal, Espoir pour la Santé, in Senegal, is an independent non-profit public service organization founded in Senegal in 1995. This research centre is specialized in infectious diseases with the aim of reducing the burden of various issues affecting rural and urban communities of West Africa.
CRB-EPLS’ role is to develop immunology laboratory capacity specific to tuberculosis research through technology transfer and training by our partner SATVI. CRB-EPLS also collects critical TB epidemiological data in Senegal to design and conduct the future Phase 3 infant efficacy multicentre trial.

The Institut Pasteur from Madagascar (IPM) created in 1898, is a non-profit private foundation under the Ministry of Public Health from Madagascar and recognized of public utility by the Government of the Malagasy Republic. This status gives IPM four main tasks: research activities directly applied to Malagasy national health priorities; Public health activities with its National Reference Centers bringing expertise to the Malagasy Ministry of Public health; Training and learning activities essential in the Malagasy context and other service activities including an international vaccine center.
The IPM research projects are aligned with national priorities and meet the challenges of international health. The focus of IPM’s fight against ID includes researching new ways of preventing and treating of chronic disease. IPM designed and developed ID surveillance which ensures quality diagnostics (IPM hosts 10 national or international (WHO) reference laboratories) and actively intervenes in the epidemic response.
IPM is participating in the WP2 of the MTBVAC project for a capacity building in detecting Mycobacterium tuberculosis infection and lead the WP3 to gather information about prevalence rates of TB infection and disease in Madagascar and Senegal to prepare for future vaccine efficiency trials.
IPM will provide their local immunological, clinical and epidemiological expertise to develop their site to a prospective late stage TB vaccine clinical trial site.
TCD Global
TCD is a Contract Research Organisation (CRO) headquartered in Centurion (Pretoria), South Africa, providing end-to-end clinical development services. Founded in February 2000, initially as a niche African CRO focused primarily on regulatory and monitoring services.
TCD will act for and on behalf of Biofabri to assist in the conduct of the clinical trial.
TCD is a Contract Research Organisation (CRO) headquartered in Centurion (Pretoria), South Africa, providing end-to-end clinical development services. Founded in February 2000, initially as a niche African CRO focused primarily on regulatory and monitoring services.
TCD will act for and on behalf of Biofabri to assist in the conduct of the clinical trial.
A Quality Management Plan will be in place to ensure the quality and integrity of the data, which will be monitored by TCD.
TCD will monitor protocol adherence and perform trial data monitoring and management, statistical analysis, quality assurance and preparation of ICH-GCP compliant study reports and their submission to Regulatory Agencies, in cooperation with Biofabri.